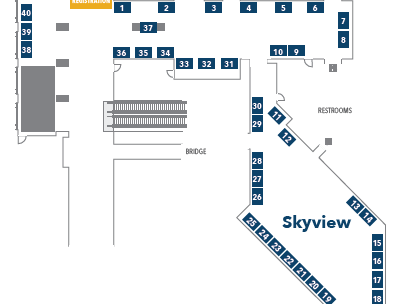
The Food Safety Consortium will take place October 20-22, 2024, at the Crystal Gateway Marriott, Arlington VA directly across the Potomic River from Washington, DC. The Program starts with several pre-conference workshops and training which leads into two full days of high-level panel discussions and educational presentations.
Organized by Food Safety Tech and the American Frozen Foods Institute (AFFI) the Food Safety Consortium Conference has been an educational and networking event since 2012 for Food Protection that has food safety, food integrity and food defense as the foundation of the educational content of the program.
CBs, Auditors, Food Companies, plan to attend the FSSC Insights Event, North America 2024 at the The Food Safety Consortium, Tuesday October 22, 2024, at the Crystal Gateway Marriott, Arlington VA. See the agenda for program details.
Use the interactive Agenda below. 1) Click on the session title and see a detailed description of the session along with the speakers and panelists. 2) Use the Tag filter to narrow down your search of topics.
Make it Conference to Remember! Bring the Family, enjoy the sights of our nation's capital and prepared to be wowed by the learning opportunities.
As Chief Product Officer at Trustwell, Todd is responsible for the product lifecycle for Genesis Foods and FoodLogiQ product lines, including feature ideation, prioritization, and delivery of new releases.
No records found.
Gold Standard Diagnostics Horsham
GSD is a leading global provider of rapid diagnostic test kits and instrumentation for Food Pathogens, Allergens, Mycotoxins, Glyphosate & GMOs. The product lines include ELISA, PCR, Microgen kits, lateral flow strips, BACGro Culture Media and more.
Smart Food Safe provides cutting-edge software solutions that enable businesses to effectively manage all aspects of Quality, Food Safety, Regulatory, and Traceability.
QIMA/WQS offers food safety and audit solutions, from farm to fork through GFSI-recognized certifications (PrimusGFS, GLOBALG.A.P., SQF, and BRCGS) food certification schemes, sustainable protocols, inspections, and training.
At FOAMit, we design and build the best foam cleaning equipment, supported by exceptional customer service. Whatever you need to do – foam, fog, spray, gel, or more – our equipment solutions can help.
Bureau Veritas is a global leader in Testing, Inspection, and Certification in the areas of quality, health, safety, environment, and social responsibility. Visit our team at booth 23!
SCS Global Services is a leading third-party certification body specializing in food safety and sustainability certifications, as well as training, and consulting services. SCS’ certification services include: SQF, BRCGS, Non-GMO Project, Organic & more.
A global leader in food safety consulting, TAG assists companies in areas such as Recall/Crisis Management, Food Defense, Supply Chain, Food Safety Culture & Regulatory Compliance.
Eagle Protect supplies independently tested & verified gloves to eliminate risks to your food safety practices. Our traceable supply chain ensures unrivaled performance, compliance & protection.
We are SGS – the world’s leading testing, inspection and certification company. We are recognized as the global benchmark for sustainability, quality and integrity.
Allera is the most user-friendly digital operating system for food safety and quality team to go digital, automate supplier management, unlock data, and streamline document control.
Neogen Analytics tracks and unifies the workflow and data generated within your environmental monitoring, product testing, and sanitation verification to improve quality and decrease risk while streamlining operations.
No records found.

Payment Method: Credit Card or PayPal. PayPal is our credit card processor. You can pay with your credit card and you do not need a PayPal account. Please note, the merchant name on your credit card statement will be "Innovative Publishing"
What are the Registration Prices:
We are happy to welcome everyone to the DC area for the 2024 Food Safety Consortium and Canninoid Quality Conference. We also understand not all travel budgets are created equal and keeping that fact in mind, we have simplified our registration prices!
We are Offering On-Site Individual registrations as well as discounts for teams of 3 or more participants from the same company.
All On-Site Attendees have full access to all Consortium sessions, including the Cannabinoid Quality Track. For your registration fee, you will be able to participate in 30+ carefully curated educational sessions, panels and TechTalks.
We are also offering a number of Pre-Conference Trainings and Workshops (Sunday) for an additional fee, that you will be able to add to your registration.
* FSC/CQC Individual Registration - In Person On-Site:
Early Bird - $795 - Available until June 30, 2024
Advanced - $995 - Available between July 1 - Oct.6, 2024
Late/On-Site - $1195 - Available Oct. 7, 2024
This is a Single Participant registration. The On-Site option provides you the ability to be present in "the room when it happens," and engaging with colleagues and vendors. This registration allows you access to all the Conference content on-site, including the Cannabinoid Quality Track. This registration DOES NOT INCLUDE the PAID pre-conference Workshops on Sunday.
When you register 3 or more participants from your company, you will receive a 50% discount per registration AFTER the 3rd REGISTRATION. Please note that in order to receive this discount, ALL REGISTRATIONS MUST BE MADE AT THE SAME TIME. There will be NO retroactive refunds.

More details for each are on the agenda
* Innovative Environmental Monitoring: Revolutionizing Listeria monocytogenes Detection with a Three-Class Sampling Plan
During this 1/2-day workshop, attendees will gain insights on a groundbreaking approach to environmental monitoring, specifically targeting Lm, designed to address the deficiencies of traditional methods.
* Unveiling the Source: Investigating the Causes of Food Safety Events
Join us for an interactive workshop where we delve into the intricacies of root cause analysis and its pivotal role in determining the causes of adverse food safety events. Through real-world cases studies and practical exercises, participants will gain valuable insights into how information gathered through root cause analysis can inform effective solutions and pave the path forward for preventing similar events in the future.
* Unraveling Genetic Mysteries: Exploring Whole Genome Sequencing and Next Generation Sequencing in Food Safety
Join us for an enlightening workshop session as we delve into the revolutionary world of Whole Genome Sequencing (WGS) and Next Generation Sequencing (NGS) technologies and their pivotal role in advancing food safety efforts. Explore the concept of Single Nucleotide Polymorphisms (SNPs) and discover the magic number of SNPs that determines a genetic match. Learn about the applications of these technologies in the food industry and their implications for regulatory compliance.
* Advanced Food Safety HACCP Workshop
This 20-hour workshop focuses on the maintenance and continuous improvement of effective Food Safety HACCP Programs. Experienced and knowledgeable instructors have chosen key subject areas that are important to current issues and resolutions for effective management of Food Safety HACCP Programs. The agenda will vary based on current food safety issues, FSMA updates and the needs of the actual attendees based on their industry sector. We encourage our attendees to share their questions and areas of interest at prior to their attendance. Please submit questions to the instructors via email at contact@newslow.com. We are providing this course in three (2-3 hr.) virtual classes conducted by a live instructor in September and October culminating with an in person 8–10-hour class on October 20th at the Food Safety Tech Consortium in Arlington, VA.* FSPCA Intentional Adulteration (IA) Conducting Vulnerability Assessments Participant Course
This course will provide participants with the knowledge to implement the requirements of conducting a vulnerability assessment under the Mitigation Strategies to Protect Food Against Intentional Adulteration (IA) regulation of the U.S. Food and Drug Administration (FDA). This regulation is one of a number of regulations and guidance that implement the provisions of the 2011 Food Safety Modernization Act (FSMA).
The Mitigation Strategies to Protect Food Against Intentional Adulteration regulation (referred to as the IA rule) is aimed at preventing intentional adulteration from acts intended to cause wide-scale public health harm, including acts of terrorism targeting the food supply. The regulation requires that certain activities must be completed by a “food defense qualified individual” who has successfully completed training in the conduct of a vulnerability assessment (21 cfr 121.4). This course developed by the FSPCA is the “standardized curriculum” recognized by FDA; successfully completing this course is one way to meet the requirements for a “food defense qualified individual” responsible for conducting a vulnerability assessment.
The IA rule does not specify a particular method that you must use to conduct your vulnerability assessment. Two potential methods that can be used are the Key Activity Type (KAT) and/or the Three Fundamental Elements methods. If you conduct your vulnerability assessment using the KAT method with no modifications, you should consider completing the FSPCA IA Conducting Vulnerability Assessments Using Key Activity Types course. However, if you use any modifications to the KAT method or plan to use the Three Fundamental Elements method, then this course would provide the tools you need to do so.
18 CE Hours: The Food Safety Consortium Conference’s two-day program is recognized by NEHA (National Environmental Health Association) for 12.0 Continuing Education (CE) Hours. If you participate in one of this Pre-Conference Training and attend the conference (a total of three days), the NEHA CE Hours is 18.
There is AN ADDITIONAL registration fee for the Pre-Conference Training.
You MUST first register for the Food Safety Consortium and/or the Cannabinoids Conference on Monday and Tuesday.
* Auditor Training Workshop
Workshop Participants will be provided an in depth overview of the food safety auditing profession. 4 part series designed to provide the knowledge, behaviors and technical skills attributed to a competent food safety auditor. The series includes 3 virtual 2 hour presentations conducted by a live instructor to support participants learning experience regardless of their current level of auditing experience. These sessions are recorded and available for additional self-paced study for less experienced participants, while experienced auditors can refresh their understanding of auditing fundamentals before advancing to the more complex skills and critical thinking behaviors needed to audit high risk products. The course culminates with a full day of in person instruction on advanced topics such as potential conflicts of interest, enhanced conflict resolution techniques and providing tips in advanced written communication skills to support the delivery of comprehensive audit reports. A variety of learning techniques such as case studies and role playing will be used to reinforce the knowledge and skills covered by this course of study.
Registrants will participate in a 4 part series of virtual presentations that will culminate in the day of review class. The one day review, conducted On-Site in Washington, DC, will include case studies and role playing to reinforce the knowledge and skills covered by this course of study. Once you register for this Pre-Conference workshop, you will receive invitations to join the webinars PRIOR to arrival at the Review day.
Can I Cancel my Registration:
Cancellations minus a 20% cancellation fee are available until October 7, 2024. Cancellations MUST be submitted in WRITING to Veronica Allen, CMP, Events Manager at vallen@innovativepublishing.net. Cancellation requests MUST be received by October 7, 2024. There will be NO refunds on Cancellations received after October 7.
We will gladly substitute your registration to another colleague.
Do you have a room block at the hotel?
Attendees to the Food Safety Consortium and Canninoid Quality Conference are offered a special group rate of $257.00 + taxes per night if booked by September 27, 2024, via this link. You can also find the Discounted Hotel Block Reservation link at the top of the conference homepage at www.FoodSafetyConsortium.org.
Please note, in order to produce and host the conference at the hotel, the Food Safety Consortium and Canninoid Quality Conference has to financially guarantee the room block. We greatly appreciate it if you reserve your hotel room through the above link. Please do not use third party travel apps like Kayak, Hotel (dot com) or Marriott points to reserve your room. Those rooms are not considered part of the guaranteed Consortium room block. It takes a village to share food safety knowledge and you are doing your part presenting but also by reserving your hotel room through the room block. Thanks for your understanding.

Transportation information
The Crystal Gateway Marriott is only one mile from Reagan National Airport (DCA), two miles to Washington, DC and directly connected to the Crystal City Metro station. Ideally located in Arlington, VA, the hotel is steps to the exciting new National Landing development and Pentagon City; as well as a quick and convenient Metro ride to downtown Washington D.C. and Old Town Alexandria.
The hotel provides a complimentary Airport Shuttle, that runs daily between 5:00 AM to Midnight, every 30 minutes. You can schedule it by calling the hotel.
You can also take a metro ride from the Airport by following the signs to the National Airport Metro Station. You will need to purchase a fare care from one of the machines at the station. The fare should be ~ $2.
You can take the Yellow Line (towards Mount Vernon Square) or the Blue Line (towards Largo). Depending on the time you arrive at the airport, trains will run between 5 to 15 minutes.
The hotel is the next stop, CRYSTAL CITY Metro Station. Exit the station via the S. Clark & 18 Street exit, Hotel is a 5 minute walk from the hotel across the metro station
A typical UBER ride to the hotel from the Airport will cost you between $10 - $45 depending on the time you arrive. Be aware that rush hour in the DC area runs between 6:30 am – 9:30 am and 3:00 pm – 6:30 pm.
You can also board a TAXI from the taxi stand at the airport. Fare should run you about $15.
You can also take Amtrak/ACELA trains to Union Station in DC and take the Metro RED Line (toward Shady Grove). Change trains to the YELLOW Line (toward Huntington) and get off at the Crystal City Station. Exit the station via the S. Clark & 18 Street exit, Hotel is a 5 minute walk from the hotel across the metro station.
OTHER AREA HOTELS
If you still need to make hotel arrangements, or extend your stay, for the Food Safety Consortium, search for availability through our travel partner, aRes Travel.
aRes Travel is a third-party travel planner. Rates, deposits, and cancellation policies may vary and are the responsibility of the guest. Questions on hotel policies or payments made on aRes website should be directed to the aRes Reservation Center or to the hotel directly.
aRes Travel Link (With Event Dates Populated) - https://bit.ly/3zI38LV
MAKE THE MOST OF YOUR TRAVEL TO THE NATION'S CAPITAL
DC has lots to offer! Here are a couple of things for you to explore:
If you would like to visit the National Air & Space Museum, make sure to make reservations in advance. Entrance is free but timed-tickets are needed to be able to enter the museum.
You can make reservation via this link
To visit the National Museum of African American History, you will need to make ticket reservations in advance. There are limited tickets and they tend to be gone pretty fast. Make sure to make reservations at least a month in advance via this link
To visit the Holocaust Memorial Museum, you can reserve timed tickets via this link
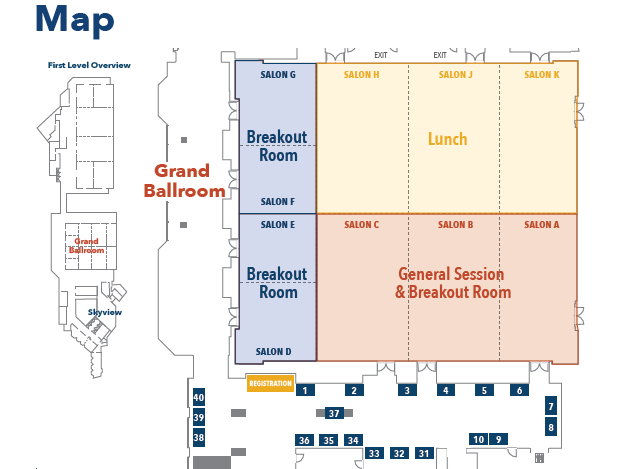
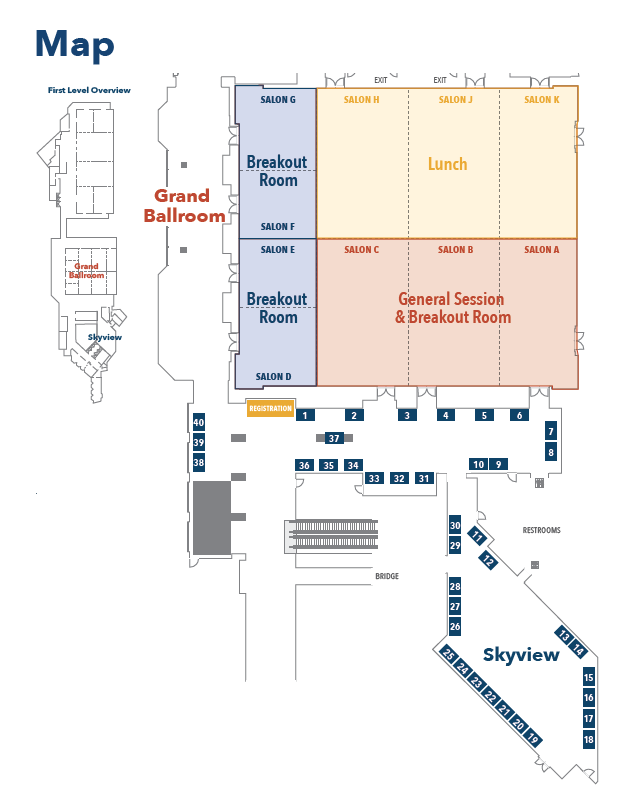
Floor Plan for Sessions and Lunch